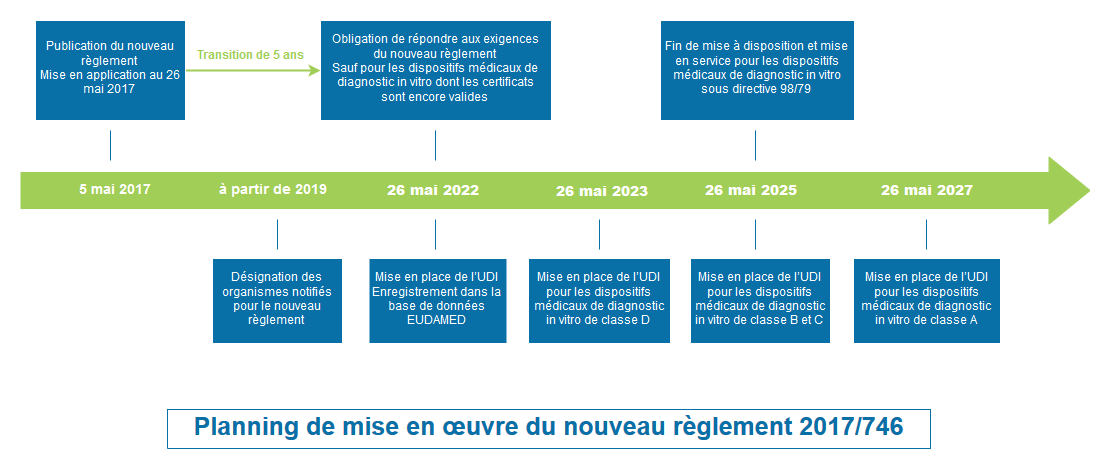
Entry into force and dates of application (Article § 113)
- Published in the Official Journal of the European Union on 5 April 2017
- Entry into force on 25 April 2017
- Compulsory application from 26 May 2022
Certificates issued by notified bodies (or self-declarations) in accordance with directives 98/79/CE after 25 April 2017 remain valid until the end of the period indicated on these certificates and cannot exceed five years. They are, nevertheless invalidated at the latest by 27 May 2024.
Attention
- Classification rules for IVD change under Regulation 2017/746. A number of self-declared IVD with CE marking under Directive 98/79/EC will have to be certified by a Notified Body under Regulation 2017/746.
- Certain provisions apply before the date of 26 May 2022, even for self-declared devices with CE marking under Directive 98/79/EC.
The dates of application in detail
You will be able to continue to market your devices under the Directive while your certificates of conformity are valid, even if this comes after the mandatory date of application of the Regulation on 26 May 2022. But this extension is only possible under certain conditions:
- Not implementing any significant change in the design of the device or its purpose.
- Continuing to comply with the requirements mentioned in Directive 98/79
However, the “other” in vitro diagnostic medical devices may no longer be released on the market by virtue of the Directive after 26 May 2022: your declarations of conformity with the Directive will no longer be valid after this date!
Therefore, it will be necessary to make the transition before 26 May 2022 for this type of device.
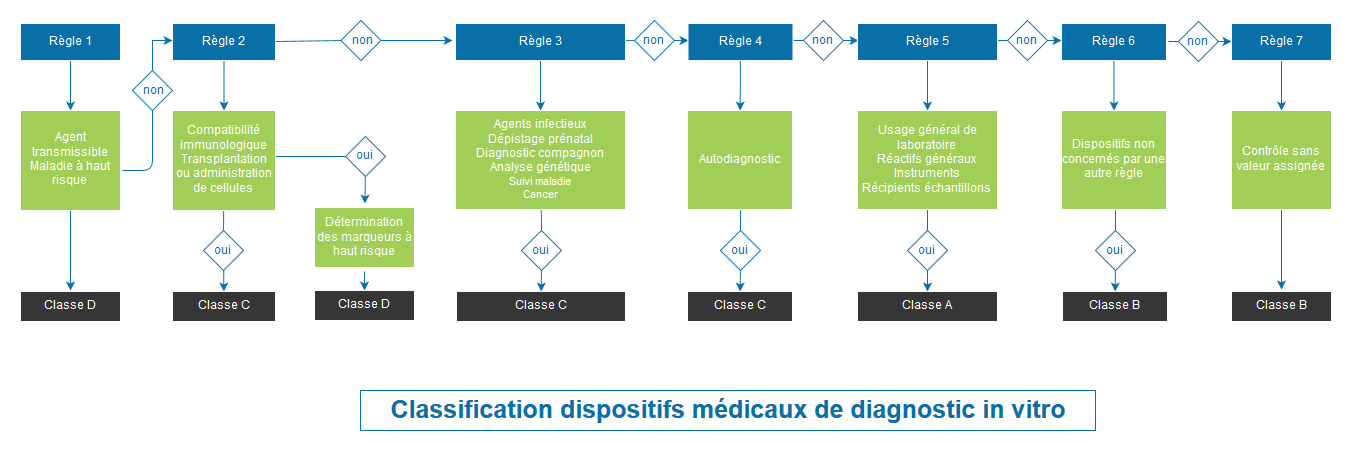
CLASSIFICATION OF MEDICAL DEVICES ACCORDING TO REGULATION 2017/746
Regulation 2017/746 completely overhauls the classification system. Classification is based on the risk for the individual and public health. The Regulation provides for 4 classes of devices: A (lowest risk class), B, C and D (highest risk class).
Class B, C and D devices, as well as sterile Class A devices, will be submitted for evaluation by a notified body for their CE marking.
Therefore, this is a major development and it is essential to determine the class of your devices according to Regulation 2017/746 as soon as possible in order to anticipate the impacts on your products and your organisation.