Entry into force and dates of application (Article § 113)
- Published in the Official Journal of the European Union on April 5, 2017
- Entered into force on April 25, 2017
EU Regulation 2017/746 (IVD) entered into force on April 25, 2017 and will be applied on May 26, 2022. However, transitional provisions have been adopted to avoid a shortage of MD-DIVs and to allow manufacturers to comply with the new regulatory requirements. These transitional provisions have made it possible to extend, under certain cumulative conditions, the validity of the CE certificates of legacy devices (devices already CE marked under the directive).
For IVDs, these deadlines extend until:
- December 31, 2027 for class D,
- December 31, 2028 for class C, and
- December 31, 2029 for sterile class B and A.
This extended marketing period is only possible under certain conditions:
- Not implementing any significant changes to the design or purpose of the device
- Continue to comply with the requirements mentioned in Directive 98/79/EC
For devices classified as “other” under the Directive and classified as “A” under Regulation EU 2017/746, the provisions of the Regulation have been applicable since May 26, 2022. These devices can therefore no longer be placed on the market under the Directive since May 26, 2022.
Please note
- Classification rules for IVD change under Regulation 2017/746. A number of self-declared IVD with CE marking under Directive 98/79/EC will have to be certified by a Notified Body under Regulation 2017/746.
- Certain provisions apply before the date of 26 May 2022, even for self-declared devices with CE marking under Directive 98/79/EC.
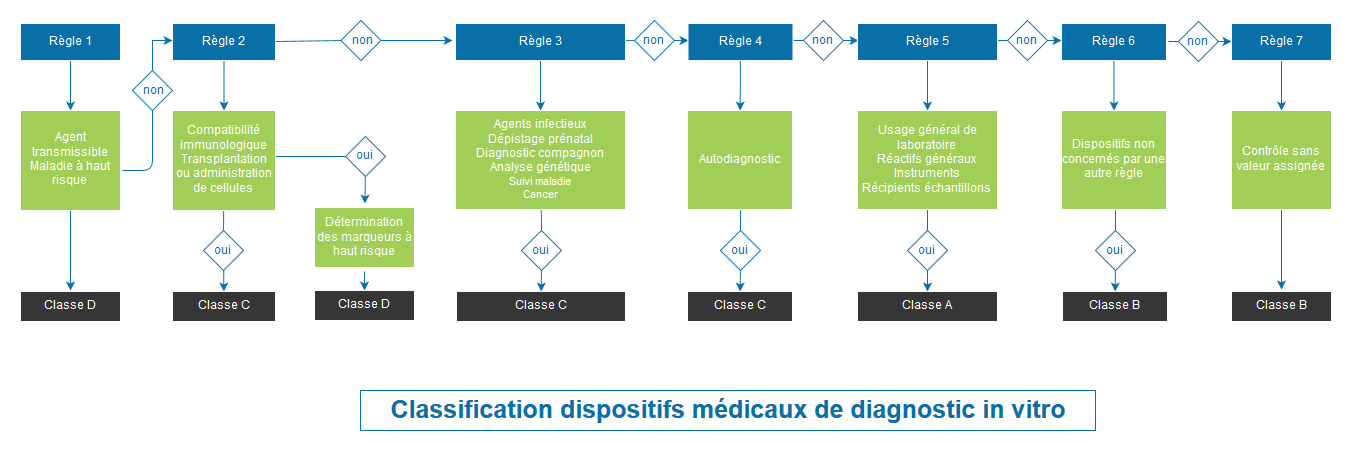
CLASSIFICATION OF MEDICAL DEVICES ACCORDING TO REGULATION 2017/746
Regulation 2017/746 completely overhauls the classification system. Classification is based on the risk for the individual and public health. The Regulation provides for 4 classes of devices: A (lowest risk class), B, C and D (highest risk class).
Class B, C and D devices, as well as sterile Class A devices, will be submitted for evaluation by a notified body for their CE marking.
Therefore, this is a major development and it is essential to determine the class of your devices according to Regulation 2017/746 as soon as possible in order to anticipate the impacts on your products and your organisation.