For medical devices and in vitro diagnostic medical devices, CE marking means that products comply with the essential safety requirements of regulations (2017/745 on medical devices and 2017/746 on in vitro diagnostic medical devices)
According to the class of the devices, CE marking is awarded by a European notified body (see list of these bodies) or via a self-declaration (class I medical devices or class A in vitro diagnostic medical devices).
Also, it is almost always essential to set up a quality system within the company that complies with the requirements of standard ISO 13485 version 2016
CE MARKING OF MEDICAL DEVICES ACCORDING TO REGULATION 2017/745
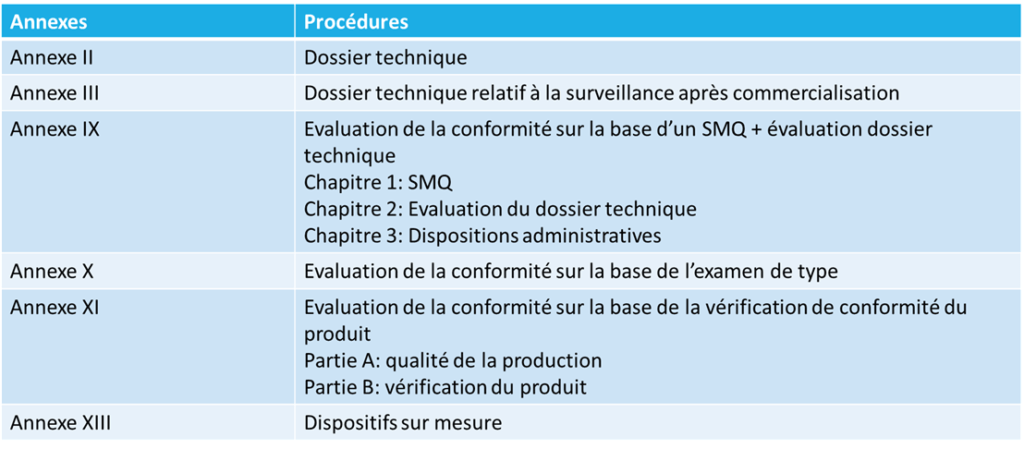
According to the class of the medical device, the manufacturer can opt for compliance with requirements according to annexes IX, X, XI or XIII with annexes II and III for the technical dossier and the PostMarket surveillance.
CE MARKING OF IN VITRO DIAGNOSTIC MEDICAL DEVICES ACCORDING TO REGULATION 2017/746
According to the class of the medical device, the manufacturer can opt for compliance with requirements according to annexes IX, X, XI or XIII with annexes II and III for the technical dossier and the PostMarket surveillance.