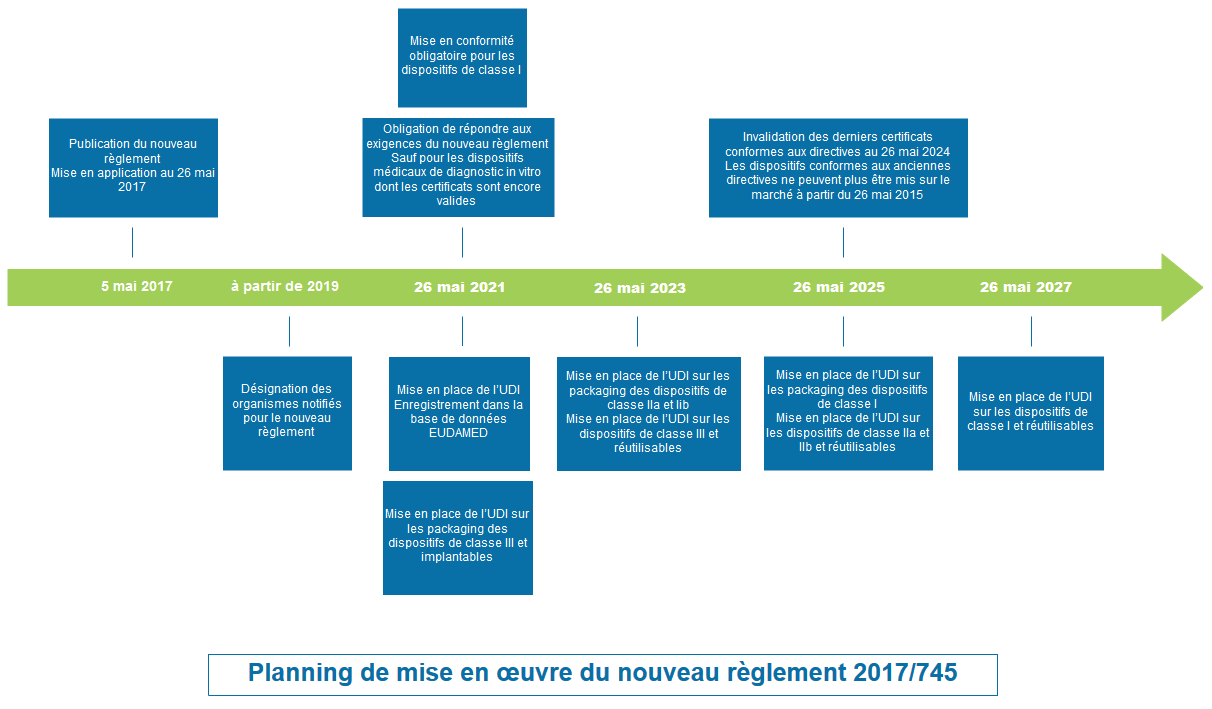
Implementation dates (Article § 120)
- Published on May 5, 2017
- Entered into force on May 26, 2017
Regulation EU 2017/745 entered into force on May 26, 2017 and has been in force since May 26, 2021 However, transitional provisions have been adopted to avoid a shortage of MDs and to allow manufacturers to comply with the new regulatory requirements. These transitional provisions have made it possible to extend, under certain cumulative conditions, the validity of the CE certificates of legacy devices (devices already CE marked under the directive).
For medical devices, these deadlines extend until :
- December 31, 2027 for class III devices and class IIb implantable medical devices
- December 31, 2028 for other class IIb medical devices, class IIa devices, and class I devices placed on the market in a sterile state or having a measuring function.
This extension of the placing on the market is only possible under certain conditions
- Not implementing any significant changes to the design or purpose of the device
- Continuing to comply with the requirements mentioned in Directive 93/42/EEC
For class I medical devices (which remain in class I under EU Regulation 2017/745), the provisions of the Regulation have been applicable since May 26, 2021. These devices can therefore no longer be placed on the market under the Directive since May 26, 2021.
Schedule for the implementation of regulation 2017/745
- In this schedule, see also the implementation of the UDI (Unique Device Identification)
- Registration in the EUDAMED database
- Setting of UDI on packaging
- Setting of UDI on devices
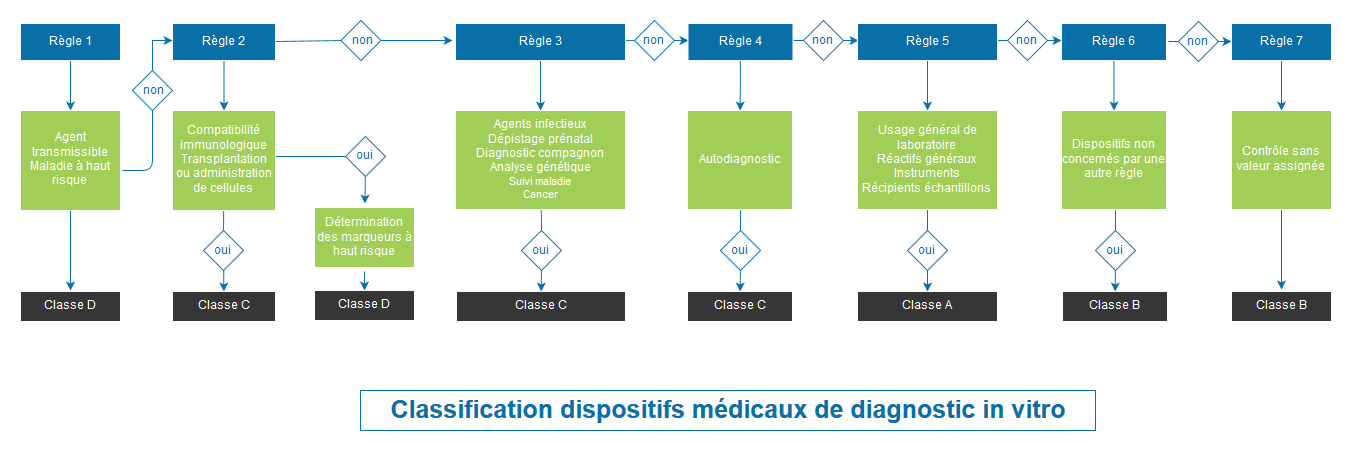
CLASSIFICATION OF MEDICAL DEVICES ACCORDING TO REGULATION 2017/745 ANNEX VIII
New indications:
In calculating the duration , continuous use shall mean:
- the entire duration of use of the same device without regard to temporary interruption of use during a procedure or temporary removal for purposes such as cleaning or disinfection of the device. Whether the interruption of use or the removal is temporary shall be established in relation to the duration of the use prior to and after the period when the use is interrupted or the device removed; and
2. the accumulated use of a device that is intended by the manufacturer to be replaced immediately with another of the same type
A device is considered to allow direct diagnosis when it provides the diagnosis of the disease or condition in question by itself or when it provides decisive information for the diagnosis.
NEW RULEs
Rule 11: Software designed to provide data used to make therapeutic or diagnostic decisions belong to class IIa, unless these decisions have an effect which may cause:
- Death or an irreversible deterioration in the person’s state of health, in which case it belongs to class III, or
- A serious deterioration in the person’s state of health or a surgical procedure, in which case it belongs to class IIb.
Software intended to monitor physiological processes is classified as class IIa, except if it is intended for monitoring of vital physiological parameters, where the nature of variations of those parameters is such that it could result in immediate danger to the patient, in which case it is classified as class IIb.
All other software is classified as class I.
Rule 19: All devices incorporating or consisting of nanomaterial are classified as:
- Class III if they present a high or medium potential for internal exposure,
- Class III if they present a low potential for internal exposure,
- Class IIa if they present a negligible potential for internal exposure.
Rule 20: All invasive devices with respect to body orifices, other than surgically invasive devices, which are intended to administer medicinal products by inhalation are classified as class IIa, unless their mode of action has an essential impact on the efficacy and safety of the administered medicinal product or they are intended to treat life-threatening conditions, in which case they are classified as class IIb.
Rule 21: Devices that are composed of substances or of combinations of substances that are intended to be introduced into the human body via a body orifice or applied to the skin and that are absorbed by or locally dispersed in the human body are classified as:
- Class III if they, or their products of metabolism, are systemically absorbed by the human body in order to achieve the intended purpose
- Class III if they achieve their intended purpose in the stomach or lower gastrointestinal tract and they, or their products of metabolism, are systemically absorbed by the human body
- Class IIa if they are applied to the skin or if they are applied in the nasal or oral cavity as far as the pharynx, and achieve their intended purpose on those cavities, and
- Class IIb in all other cases.
Rule 22: Active therapeutic devices with an integrated or incorporated diagnostic function which significantly determines the patient management by the device, such as closed loop systems or automated external defibrillators, are classified as class III.